News & Updates
PTC Therapeutics Provides Regulatory Update on Translarna™ February 12, 2026 WARREN, N.J., Feb. 12, 2026 /PRNewswire/ -- PTC Therapeutics, Inc....
26 Feb, 2026
Friday, 28 November 2025, 9AM - 5:00PM Level 3, 48 Flemington Road, Parkville, Vic A one-day professional learning workshop for...
04 Nov, 2025
First returning adult patient from Phase 1b study recently dosed; additional returning patients being scheduled 11-month open-label study will evaluate...
03 Nov, 2025
Genetics Webinar Series - Events WEBINAR 1 Could It Be Genetic? If you've ever wondered whether there might be a...
08 Sep, 2025
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Jul. 21, 2025-- Sarepta Therapeutics, Inc. (NASDAQ:SRPT), the leader in precision genetic medicine for rare diseases, today issued...
25 Jul, 2025
– New open label data in Becker demonstrated sustained disease stabilization up to three years, reinforcing prior clinical findings –...
27 Jun, 2025
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Jun. 15, 2025-- Sarepta Therapeutics, Inc. (NASDAQ:SRPT), the leader in precision genetic medicine for rare diseases, today provided...
22 Jun, 2025
Dear Members of the Duchenne community,We are excited to share positive data following 48 weeks of dosing in the FORWARD-53...
10 May, 2025
The World Duchenne Organization is thrilled to announce that registrations are now open for the Duchenne Care Conference 2025. The...
28 Apr, 2025
CAMBRIDGE, Mass., March 18, 2025 -- Sarepta Therapeutics, Inc. (NASDAQ:SRPT), the leader in precision genetic medicine for rare diseases, shared...
19 Mar, 2025
Dyne Therapeutics Announces New Long-Term Clinical Data from Phase 1/2 DELIVER Trial of DYNE-251 in Duchenne Muscular Dystrophy Demonstrating Unprecedented...
18 Mar, 2025
- Trial met primary endpoint of reduction in circulating levels of creatine kinase (CK), a biomarker associated with skeletal muscle...
19 Dec, 2024
Dear Members of the Duchenne Community, Today, Percheron Therapeutics, a public company registered in Australia and focused on developing treatmentsfor...
19 Dec, 2024
Australian Government bans genetic discrimination in life insurance: A big win for preventive health Following the recommendations of a report led...
11 Sep, 2024
Philips after consultation with TGA is conducting an Urgent Product Defect Correction for the Philips BiPAP A40 Pro Ventilator, BiPAP...
30 Jul, 2024
Philips after consultation with TGA is conducting an update to the Urgent Product Defect Alert of Philips BiPAP A30, BiPAP...
04 Jun, 2024
Pfizer gene therapy clinical trials commence in Australia! The first Australian boy has been screened as part of the Pfizer...
06 Sep, 2022
The long awaited Federal Parliamentary Inquiry report into Approval Process for New Drugs and Novel Technologies has now been released....
15 Dec, 2021
Save Our Sons Duchenne Foundation has just written a submission on behalf of our community to the Senate Standing Committee...
25 Oct, 2021
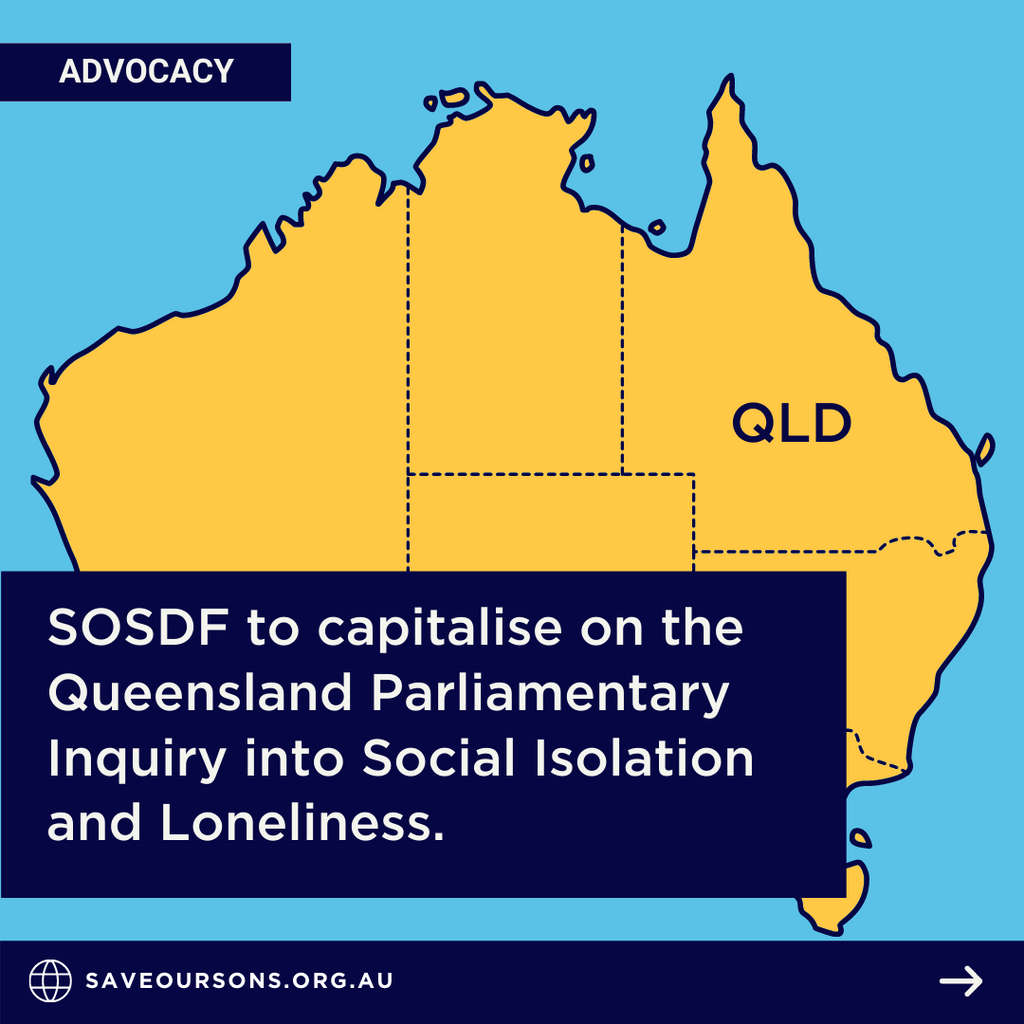
Save Our Sons Duchenne Foundation (SOSDF) thanks all Queensland families and young people who have participated in our recent consultation...
19 Aug, 2021
Are you on the Australian Neuromuscular Disease Registry (ANMDR)? With an increasing number of pharmaceutical companies interested to run clinical...
28 Jun, 2021
Sarepta Therapeutics has shared an update about themulti-ascending dose clinical trial of SRP-5051 for patients with Duchennemuscular dystrophy who are...
04 May, 2021
Sarepta Therapeutics has, last week, announced thatthey will be hosting a webcast and conference call to present resultsfrom the 30...
03 May, 2021
Dear members of the Duchenne and Becker community. You may recall that just prior to Christmas we advised of...
12 Jan, 2021
You may be aware that the National Disability Insurance Scheme (NDIS) is currently undertaking a consultation process with NDIS participants...
14 Dec, 2020
Yesterday, Save Our Sons Duchenne Foundation hosted an NDIS Information Session. The aim of the session was for families to...
01 Dec, 2020
Today, Save Our Sons Duchenne Foundation hosted a Carriers Information Session. The aim of the session was to provide an...
26 Nov, 2020
SOSDF would like to thank all parents and carers who recently participated in our consultation process reviewing health and hospital...
19 Nov, 2020
Murdoch Children’s Research Institute (MCRI) - Royal Children’s Hospital Melbourne Funding towards the position of Neuromuscular Clinical Trials Staff Specialist...
04 Nov, 2020
Catabasis Pharmaceuticals has announced the end of the Phase 3 PolarisDMD trial of edasalonexent in Duchenne muscular dystrophy. Edasalonexent is...
27 Oct, 2020
Save Our Sons have lodged a comprehensive submission to this most important Parliamentary Inquiry. As the peak body representing the...
20 Oct, 2020
Save our Sons Duchenne Foundation have awarded the inaugural Alex Scollard Memorial PhD Scholarship to Dr Rajiv Wijesinghe. Dr Wijesinghe will...
12 Oct, 2020
The Save Our Sons Duchenne Foundation Nurses Program was developed to ensure “Best Practice” clinical care for all with...
30 Sep, 2020
Save Our Sons Duchenne Foundation commissioned Marguerite Botha, an experienced NDIS Support Coordinator, to write a National Disability Insurance Scheme...
29 Sep, 2020
Save Our Sons Duchenne Foundation will welcome Shadow Minister for the National Disability Insurance Scheme (NDIS), the Hon Bill Shorten...
23 Sep, 2020
Save Our Sons Duchenne Foundation Nurses Program Perth Children’s Hospital - Exclusive Neuromuscular Nurse The Save Our Sons Duchenne Foundation...
17 Sep, 2020
WE NEED YOUR PERSONAL STORIES… As reported recently, the Federal Government has established a Parliamentary Inquiry into new drugs and...
10 Sep, 2020
SOSDF thanks the Duchenne and Becker community for your active participation and critical contribution to our wide ranging submission to...
09 Sep, 2020
Due to the full implementation of the National Disability Insurance Scheme (NDIS) coming to fruition across all Australians States and...
08 Sep, 2020